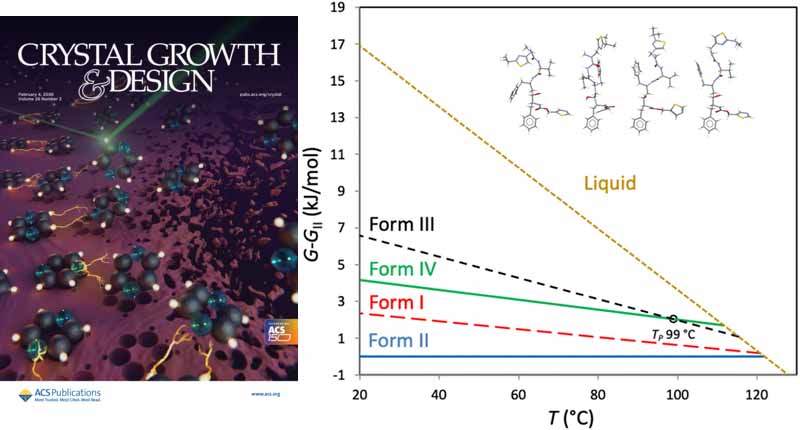
The crystal structure of Ritonavir Form IV is reported. The structure was determined by electron diffraction, and the molecular conformation and hydrogen bonding show similarities to those of Forms I and III. Form II is shown to be the most thermodynamically stable of the four known polymorphs.
Polymorphism means a solid material can form different crystal structures. Each structure has different physical properties. For pharmaceutical drugs, the crystal structure affects how the drug is made, how it is released in the body, and how long it stays stable in storage. Scientists need to figure out which crystal form is most stable and how the different forms can change into each other.
Years ago, a drug recall crisis involving ritonavir (RTV) showed how important it is to understand these different crystal forms. Scientists are still discovering new forms of RTV. The structure of RTV Form IV was recently identified using electron diffraction.
The hydrogen bonds in Form IV are similar to those in Forms I and III. Scientists determined how stable each of the four known forms is compared to the others. Forms IV and III can reversibly interconvert at about 99°C. The other forms have one-way relationships – they can only convert in one direction. Form II is the most stable version overall.
Key Terms
- Enantiotropic = two forms that can reversibly convert at a specific temperature
- Monotropic = one form is always more stable than another